The Food and Drug Administration has issued a nationwide recall for the birth control pills Mibelas 24 Fe. The recall was due to a packing error involving a specific lot of pills.
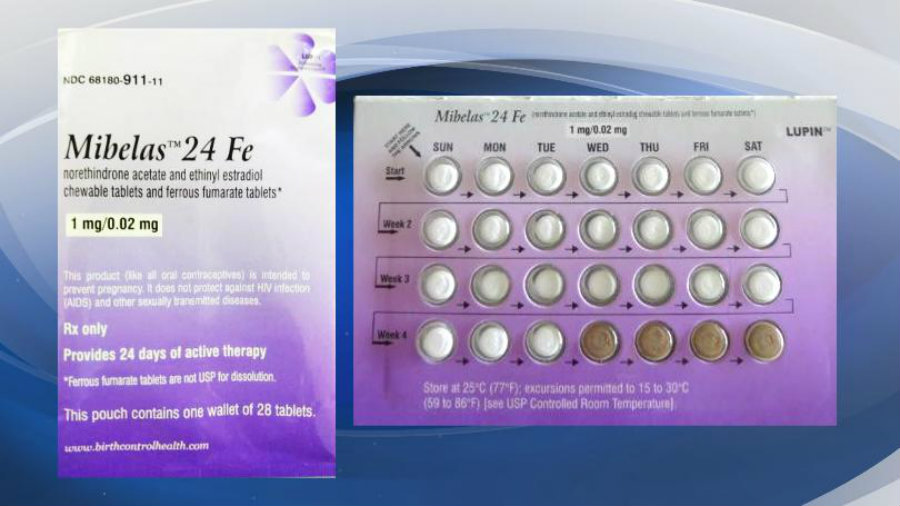
According to the FDA, there is nothing wrong with the pills. The problem is the package they are in. This might lead to undesired pregnancies, though many women might not notice any difference at all. The lot that is being recalled is the L600518 with an expiration date of May 2018.

Women are at risk of unwanted pregnancies with Mibelas 24 Fe
According to the FDA, Lupin Pharmaceuticals decided to voluntarily recall its birth control tablets because women who take the pills are actually at risk of unwanted pregnancy. Those who use to take the Mibelas 24 Fe, might have noticed that the pills are not in the normal order. The package doesn’t rotate in the way it should, so women won’t take the correct pills. The placebo pills – which are the brown ones – are at the beginning of the pack instead of the end.
“For patients in whom a pregnancy is contraindicated or in whom concomitant medications may have teratogenic effects, an unintended pregnancy may cause significant adverse maternal or fetal health consequences, including death,” the FDA announcement warned.
The risks of pregnancy are real, according to the doctor Lauren Streicher, an associate professor of obstetrics and gynecology at the Feinberg School of Medicine at Northwestern University and director of the Northwestern Medicine Center for Sexual Medicine and Menopause. She says that if women take the first four pills thinking they are the ones they should take, then women are at risk of being off the pill for eight days instead of four, so the chances of pregnancy increase. She said that this situation is not the same as missing one pill in the middle of the cycle, which is not that important.
The FDA clarified that besides the packing of this lot of Mibelas 24 Fe –which also hides the date of expiration- there is nothing wrong with the actual pills.
Lupin Pharmaceutical is now notifying all distributors and customers about the recall. Women can return the tablets to the place where they bought them to get a full refund. Lupin is a recognized pharmaceutical in India. In the United States, its headquarters are located in Baltimore.
Women who took the recalled pills should “pray”
Dr. Streicher said that those women who started the Mibelas that are being recalled and have had sex, should “pray,” but they should continue to take the treatment alongside other backup contraception methods such as condoms. If they don’t have the period at the end of the pack, they should go for a pregnancy test. Nobody has reported taking pills from the wrong lot yet, or if they have had adverse effects due to them. However, it might be too soon to say it.
The FDA recommends customers who have inquiries about the recall to contact Lupin Pharmaceuticals by phone 1-800-399-2561, 8:00 am to 5:00 pm EST, Monday through Friday.
Source: TODAY
