A new study found that treating patients with a compound derived from cannabis can significantly reduce or eliminate seizures in children and young adults.
The findings were published on May 24 in the New England Journal of Medicine, where scientists explained that children and young adults with a rare and debilitating form of epilepsy known as Dravet syndrome who took doses of marijuana extract sustained half as many seizures per month as those who received a placebo.

Furthermore, 5 percent of those treated with the cannabis extract, called cannabidiol, became seizure-free during the length of the medical trial.
Cannabidiol proved to be effective for patients with Dravet syndrome
According to the Epilepsy Foundation, there aren’t any medications that can fully control seizures in children with Dravet syndrome currently. The new study is among the first to provide solid, clinical evidence to sustain a form of treatment that is becoming widespread with the legalization of medical marijuana, but which remains unregulated.
“I can’t say enough about the importance of these kinds of medical trials. People have a sense that if 10 people say it works and it’s a bad disease like cancer or epilepsy, then it’s safe to use. That’s just false,” said Dr. Orrin Devinsky, director of NYU Langone’s Comprehensive Epilepsy Center and co-lead author of the study, according to Live Science. “Just because it’s natural and just because there may be anecdotal support from people, doesn’t mean it’s effective and safe.”
Cannabidiol, or CBD, is one of the dozens of compounds in marijuana called cannabinoids. However, unlike tetrahydrocannabinol (THC), which is the main chemical in cannabis, CBD does not get users “high.” CBD is typically administered via oil form and is thought to work by interacting with receptors on nerve cells.
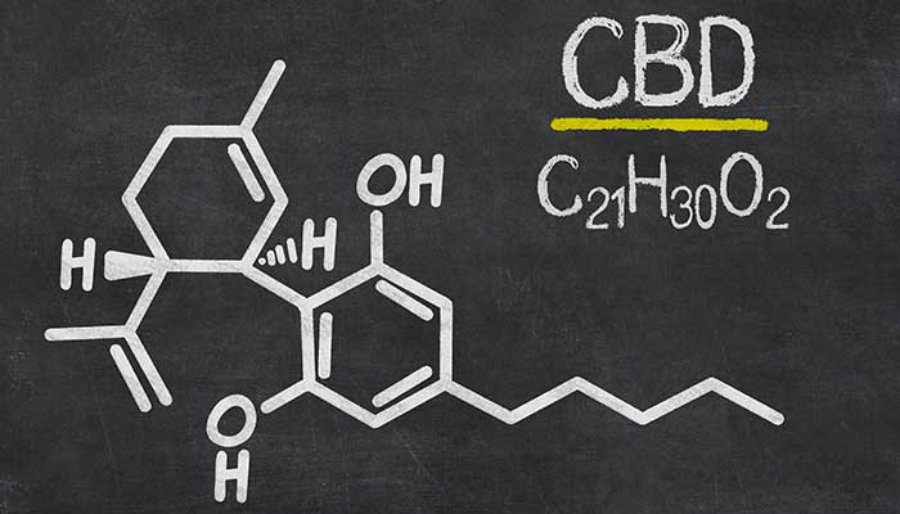
Interest in using the compound to treat epilepsy grew in 2013 when an 8-year-old girl from Colorado with Dravet syndrome showed remarkable improvement after taking CBD, which was administered by a Denver medical marijuana dispensary.
Since then, other anecdotal cases showed promise and a study from 2015, also led by Devinsky, suggested positive outcomes from the marijuana compound. That study, however, did not use a place, so results were considered to be vulnerable to a bias since doctors and patients could associate any progress to the drug.
Patients who received CBD had half as many seizures as before
For the new study, the medical trial was randomized, double-blind, placebo-controlled trial, a form of study that’s considered the gold standard for clinical research. Meaning, in the new study neither the researchers nor the patients, knew if they had been given the drug or the placebo.
The study involved 120 children and young adults, aged between 2 and 18 years, with the Dravet syndrome. Half of the patients received a placebo, and the other half received 20 milligrams per kilogram of body weight per day of the CBD drug, called Epidiolex. Epidiolex is a 99 percent cannabidiol compound manufactured by the U.K.-based company, GW Pharmaceuticals, which funded the research.
The medical trial lasted three months, and by the end, researchers compared the frequency of patient’s seizures to their seizure frequencies from a four-week period before the trial’s beginning. Those who received Epidiolex had, on average, 12 seizures per month before the study began, and after the study had ended, the frequency had dropped to six seizures per month, on average.
Most patients who took the drug showed some side effects, such as diarrhea, vomiting, fatigue and abnormal results on liver function tests, but Devinsky explained that most of these reactions were mild and could likely be reduced with an adjustment in dose.
More research is needed to assess CBD impact in other epilepsy forms
Dr. Helen Cross, a co-lead author of the new study, said it was critical to measure the effects of a drug with carefully prepared levels of CBD.
“We know exactly what’s in every single batch,” said Cross, a clinical neuroscientist at University College London’s Institute of Child Health, according to Live Science. “It’s not like the hemp oils that you can buy from the internet, which are so variable in their content.”
In the United States, CBD oil is legal (with some restrictions) in 44 states, but the substance is not fully regulated, and many patients or parents of children with epilepsy are not waiting for clinical data and are trying these unregulated versions of the compound.
Devinsky told Live Science that other studies like theirs are desperately needed, focusing in other forms of epilepsy and using other cannabis preparations. He added that it should be a priority.
Epilepsy is the fourth most common neurological condition in the world, affecting more than 65 million people globally, according to the Epilepsy Foundation. A study from April 2017 showed CBD proved to be effective in treating another rare, but severe form of epilepsy called the Lennox-Gastaut syndrome.
“The big question now is whether this drug is also effective for a larger group of people with epilepsy who don’t have these rare symptoms,” said Devinsky.
Specialists around the world believe more studies must be conducted to assess the effects of medical marijuana on epileptic patients. Dr. Sam Berkovic, a neurologist, and director of the Epilepsy Research Centre at the University of Melbourne, Australia, told Live Science that medical practice can’t be decided by anecdotes, as they are subject to many forms of bias.
Source: Live Science
